- Med Travellers
- 0 Comments
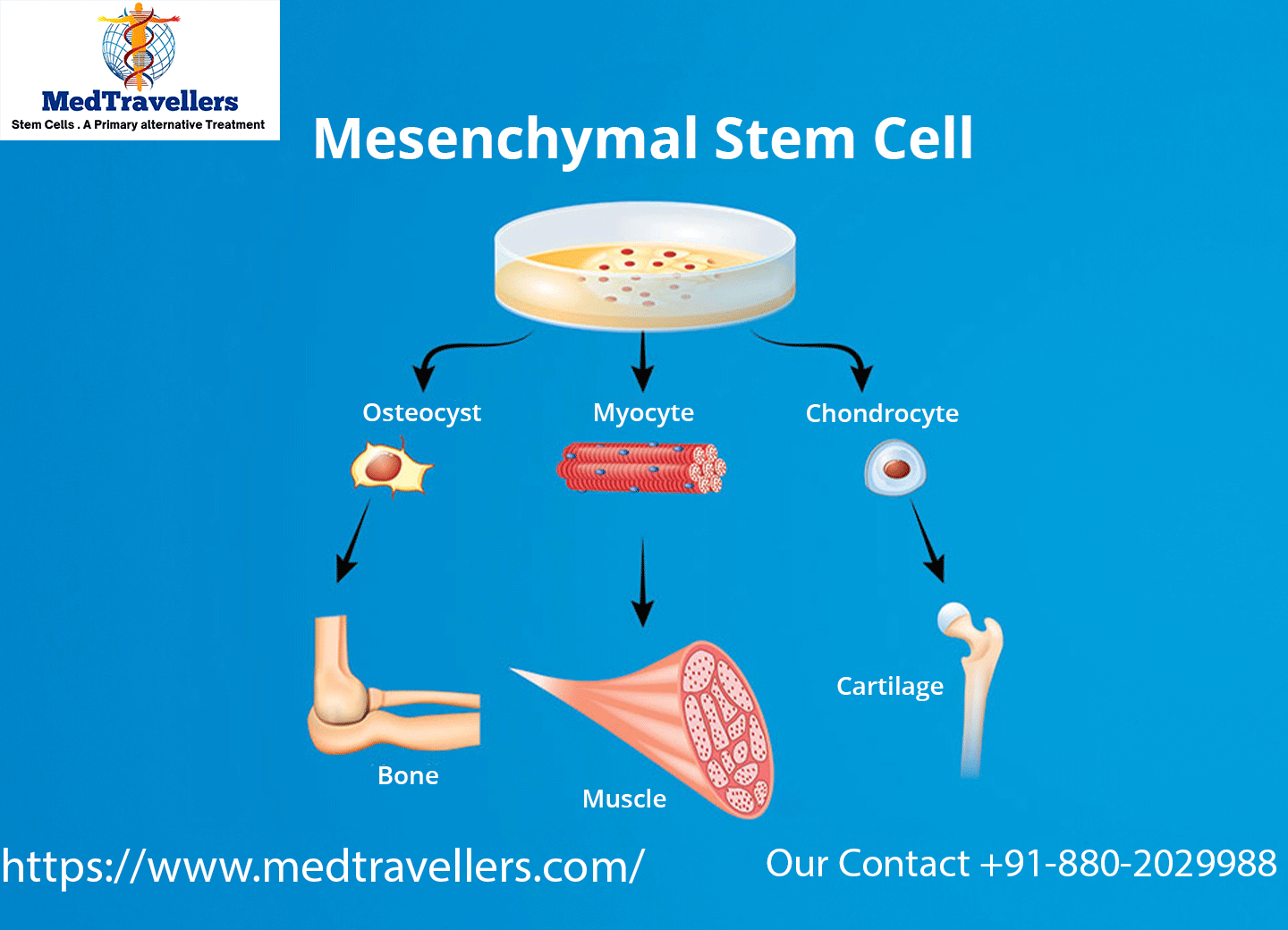
What Is a Mesenchymal Stem Cell?
Mesenchymal stem cells (MSCs) are adult stem cells that are increasingly appreciated for their potential uses in regenerative medicine. These cells can differentiate into various types of tissues, which ultimately makes MSCs a viable option to treat a wide array of illnesses and diseases affecting the human body, inclusive of but not limited to bone and cartilage injury and severe conditions related to autoimmune disease and cardiovascular conditions. MSCs are found across several locations in the body, including but not limited to the bone marrow, the adipose (fat) tissue, and the umbilical cord. Through repeated division, MSCs can assist in healing and regeneration of tissue. This represents a very helpful solution given the breadth of patients who suffer from diseases and conditions that may be poorly responsive to conventional treatment.
Features of Mesenchymal Stem Cells
The differentiating factors of MSCs are their unique characteristics, which make them an attractive target for therapeutics.
- Multipotency (Another distinguishing characteristic of MSCs is that they can differentiate into a number of different cell types, predominately those within mesodermal tissues, such as cartilage, bone, and fat cells).
- Self-Renewal (Because MSCs are able to proliferate and produce the same type of stem cells, these stem cells represent a continuous source of stem cells).
- Antigen-presenting functionality (MSCs have the ability to interact with T cells and modulate their activity, which can be beneficial for certain autoimmune diseases)
Based on these features, MSCs are a substance of ongoing research within the regenerative medicine realm.
Clinical Efficacy of MSCs in OA: White Data
Published in The Lancet was a clinical trial looking at MSC therapy for knee osteoarthritis. Patients received MSC derived from bone marrow as an injection by the doctor into the joint. Over a year the results were quite amazing. Not only did patients report less pain, but they also had very significant cartilage regeneration and improvements in joint mobility.
Key Findings:
- The majority of patients reported pain relief and improved mobility.
- MRI of treated joints demonstrated the regeneration of cartilage.
- MSCs provided more consenting and long-term non-invasive treatments than other treatment modalities applied, which included surgical involvements.
This paper highlights the potential use of MSCs as a treatment method for patients suffering from osteoarthritis, both in terms of symptomatic alleviation and regenerating damaged tissue.
MSCs and Bone Regeneration
There are excellent expectations for MSC therapy in many areas—one of which is bone regeneration. A study in Nature Reviews Rheumatology states that MSCs show great promise in treating fractures and osteoporosis. Some studies reported impressive results using MSCs harvested from adipose tissue injected into patients with non-union fractures.
In the study, greater than 80% of patients exhibited considerable bone healing with MSC treatment six months after the MSCs were injected, with greater bone density and healed fractures.
Key Findings:
- Quicker scientifically proven fracture healing with MSC-based therapy.
- Patients had improved bone mineral density, and their fractures healed faster.
- In some patients, adipose-derived MSCs healed just as well as bone marrow-derived MSCs.
MSCs in Other Clinical Applications
Beyond MSCs’ ability to regenerate bone and cartilage, these cells are currently undergoing exploration for potential treatment of other significant medical conditions:
- Autoimmune Disorders With Medical Relevance:Thanks to their immunomodulating effects and other immunological effects, MSCs are an ideal candidate to treat autoimmune disorders such as rheumatoid arthritis, lupus, and multiple sclerosis. Whether via randomized clinical trial research and clinical studies or observational cohort studies, researchers from various global locations have observed that these cells can modulate inflammation and regenerate (repair) damaged tissue.
According to a review from Stem Cells Translational Medicine, there are over 80 trials studying MSCs for musculoskeletal injury, autoimmune diseases, and several other indications.
- Cardiovascular Disorders: MSCs have been studied for their ability to heal damaged cardiac tissue after a myocardial infarction (heart attack). Research suggests that MSCs can actually improve heart function not just by replacing the damaged heart tissue, but they also provide increased ability for angiogenesis (growth of blood vessels).
- Neurological Disorders: Research is beginning to determine the efficacy of MSCs for neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease. Because MSCs can also protect nerves from injury, researchers are investigating their possible use for nerve regeneration as well.
MSCs in the Treatment of Osteoarthritis
An article published in The Lancet documenting a clinical trial evaluated the efficacy of MSC therapy in treating knee osteoarthritis. As part of the trial, patients received MSCs via intra-articular injections extracted from their bone marrow. Findings after 12 months showed significant improvements in the function of the joint, a reduction in the severity of pain, and repair of cartilage tissue. MSC-based therapies reduced inflammation and stimulated repair of tissue of the knee in patients with osteoarthritis and provided an alternative to surgical treatments.
Key Findings:
- The patients reported increased mobility and decreased pain.
- MRI imaging showed regeneration in treated joint cartilage.
- MSC injections were a long-lasting, non-surgical solution to bone-and-joint degeneration.
Use of MSCs in Bone Regeneration for Fracture Healing
Nature Reviews Rheumatology recently published a comprehensive article on using MSCs in bone regeneration, fractures, and osteoporosis. In 2020, MSCs obtained from adipose tissue were injected into patients with non-union fractures. More than 80% of patients experienced notable healing of the bone within 6 months of receiving MSCs, with improvement in bone density and a decrease in time to attain a union.
Key Findings:
- MSC therapy decreased the time taken for healing in cases of fractures.
- Patients showed results of increasing bone mineral density and improvement in fracture healing.
- In some conditions, the adipose-derived MSCs were indistinguishable from those derived from bone marrow.
Challenges in MSC Therapy
Although MSCs have great promise, there are still challenges to overcome, such as:
- Source-Differentiated Variability: There is inherent variability in the MSC characteristics based on their source tissue or patient, such as differentiation potential and proliferation rate. Therefore, there are inconsistencies in standardizing MSC therapies.
- Potential for Tumorigenesis: The likelihood to form a tumor is extremely rare, especially for autologous MSC therapies, and remains an area of great research.
- Ethics and Regulation of Use: The use of MSCs in medicine, especially MSCs from embryonic tissue, is blurred with ethical challenges in our society. Although MSC-mediated methods may yield feasible solutions to common clinical problems in the near future, the field of MSC therapies and studies is still in the early stages and has yet to be established to be safe and effective in clinical trials.
- Cost-Effective Scalability: To obtain sufficient MSCs for a clinical application in a research context or as a treatment in today’s clinical environment will be a difficult and costly endeavor. At present, researchers are working together on their productive investigations for potential low-cost production of large quantities of these cells.
Conclusion
Mesenchymal stem cells (MSCs) are emerging as a valuable and innovative resource for cell therapy and regenerative medicine in a variety of conditions, including osteoarthritis and cardiovascular disease. The regenerative potential of MSCs in tissues and their immunomodulatory function is an exciting potential therapeutic modality. While the packaging and standardization of MSCs within clinical medicine can be a complicated endeavor, research and clinical trials continue to demonstrate their promise.
FAQs
What are mesenchymal stem cells, and where can they be found?
MSCs, or mesenchymal stem cells, are adult stem cells that have the potential to differentiate into a variety of tissues, primarily bone, cartilage, and fat cells. MSCs can be found in several places in the body, including bone marrow, adipose (fat) tissue, and umbilical cord blood.
MSCs have three main properties: multipotency (becoming multiple cell types), self-renewal (an ability to differentiate continuously), and immune-modulating properties (helps regulate inflammation).
What conditions are treated with MSCs?
MSCs are being studied for promising treatments for many conditions:
- Musculoskeletal: Osteoarthritis, fractures, and other bone regeneration (clinical trials have reported considerable pain relief and cartilage regeneration in individuals with knee osteoarthritis).
- Autoimmune: Rheumatoid arthritis, lupus, and multiple sclerosis due to their anti-inflammatory properties.
- Cardiovascular: Heart injury post-heart attack and improving heart function. They also encourage the growth of new blood vessels.
- Neurological: Early studies showed improvements for assistance with both Parkinson’s and Alzheimer’s diseases. Most studies involved nerve protection and nerve regeneration.
How well documented are the MSC treatments based on clinical evidence?
Results from clinical trials show good results:
- Osteoarthritis: Patients receiving MSC injections demonstrated pain relief, better mobility, and MRI-documented articular cartilage regeneration over the course of a year.
- Bone healing: Over 80% of patients treated for non-union fractures experienced significant bone healing at 6 months after MSC therapy and increased bone density.
- Research: Currently, there are over 80 clinical trials of MSCs for multiple indications demonstrating strong scientific interest and potential.
What are some challenges and threats of MSC therapy?
- Variability: MSCs differ based on the source tissue and the individual patient and will not be uniformly standardized.
- Tumor risk: Tumor formation is very rare with the use of autologous (patient’s own) MSC and is still being studied.
- Regulatory: There are ethical issues as well as ongoing clinical trials to establish long-term risks.
- Cost/Feasibility: It is costly and technically challenging to produce enough of the MSC product for widespread clinical usage.